Modification of Reactivity by Changing Microemulsion Composition. Basic Hydrolysis of Nitrophenyl Acetate in AOT/Isooctane/Water Systems
Luis García-Río, Juan C. Mejuto, and Moisés Pérez-Lorenzo
New J. Chem., 2004,28, 988–995
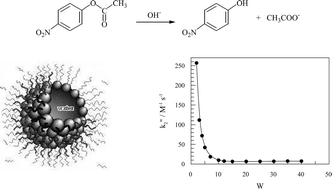
A study was carried out on the basic hydrolysis of p-nitrophenyl acetate (NPA) in AOT/isooctane/water microemulsions keeping constant the hydroxide ion concentration referred to the droplet water. The obtained results show that the pseudofirst order rate constant, kobs, is approximately 100 times lower than the corresponding value in bulk water and increases together with the [AOT], keeping constant the molar ratio W, W=[H2O]/[AOT]. On experiments varying W, keeping constant [AOT], it can be observed that kobs decreases from W=2 to W=10 as W increases, reaching a minimum value for W10, and then increasing again. The observed behavior is a consequence of two factors: the variation of hydroxide ion concentration referred to the total volume of the system, and the association of the NPA to the interface of the microemulsion. The experimental results can be explained quantitatively considering that the NPA is distributed throughout the three pseudophases of the system and that the reaction takes place solely in the aqueous microdroplet. The second order rate constant in the aqueous microdroplet of the microemulsion, kw2, increase as W decreases, showing a parallelism with the behavior observed in aqueous DMSO. The values of kw2 increase approximately 35 times from W=40 to W=2 due fundamentally to the partial desolvation of the hydroxide ions as the water content of the microemulsion decreases.